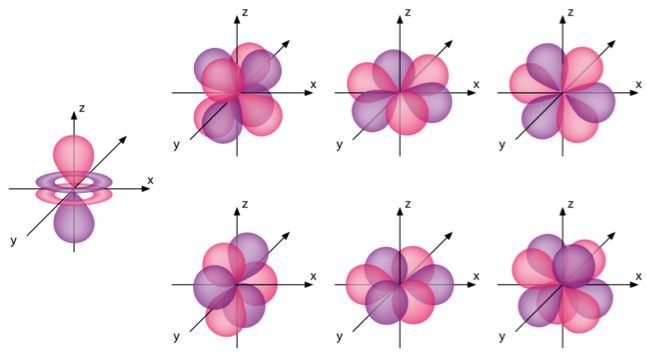Shapes Of S P D F Orbitals
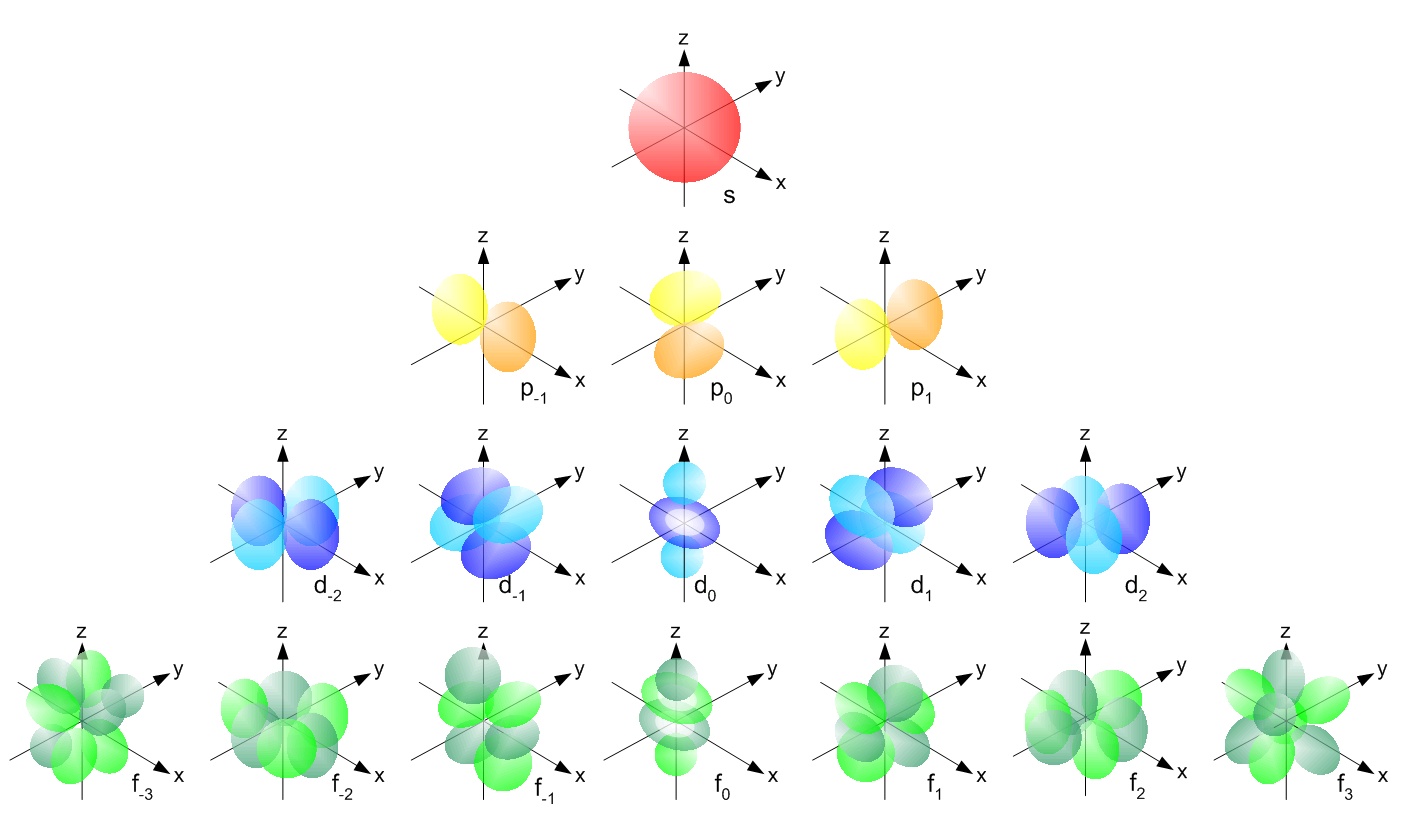
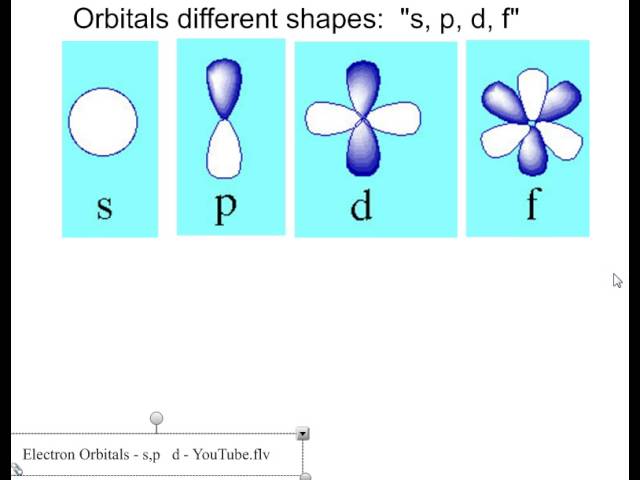
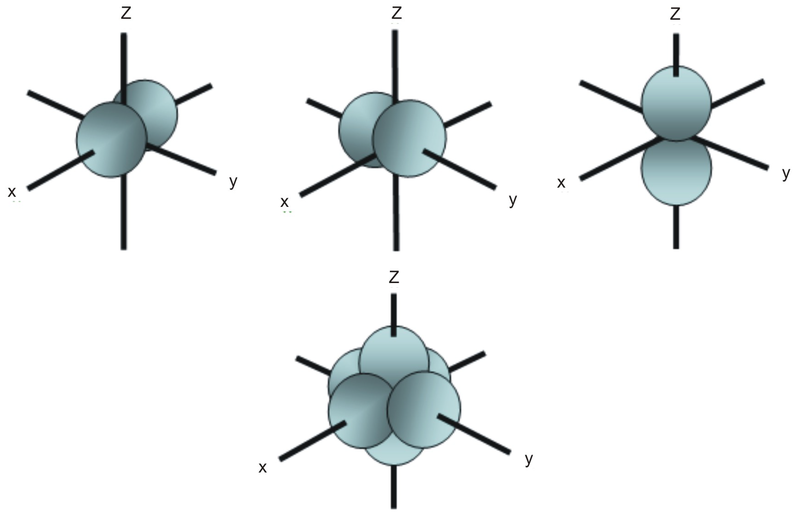
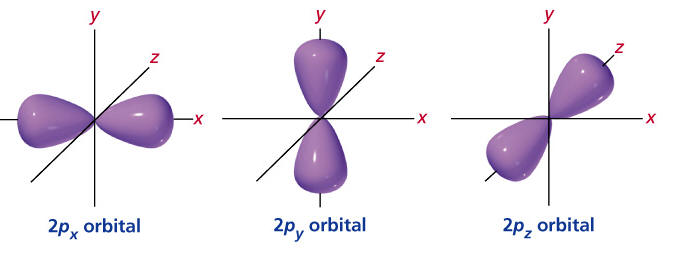
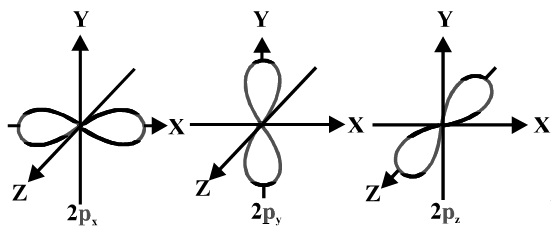
The s orbitals are spherical while p orbitals are polar and oriented in particular directions x y and z.
Shapes of s p d f orbitals. It may be simpler to think of these two letters in terms of orbital shapes d and f aren t described as readily however if you look at a cross section of an orbital it isn t uniform. These letters are s p d f g h i j and many more but here we are only looking at letters p s and d and their corresponding shapes. The three p orbitals for n 2 have the form of two ellipsoids with a point of tangency at the nucleus the two lobed shape is sometimes referred to as a dumbbell there are two lobes pointing in opposite directions from each other. S p d and f orbitals are available at all higher energy levels as well.
Learn more about atomic orbital at byjus. Letters are there to refer to the shape of the orbital. The p orbitals of higher energy levels have similar shapes although their size are bigger. Orbitals chemistry s p d and f orbital atomic orbitals are of four different kinds denoted s p d and f each with a different shape.
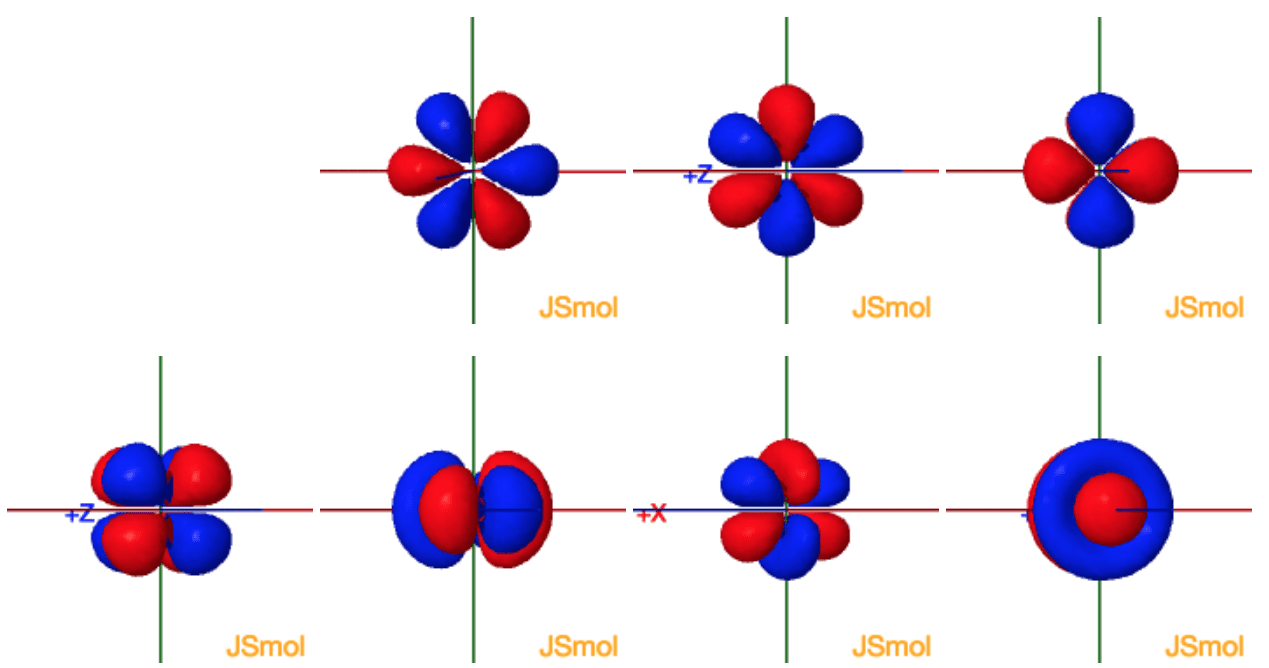
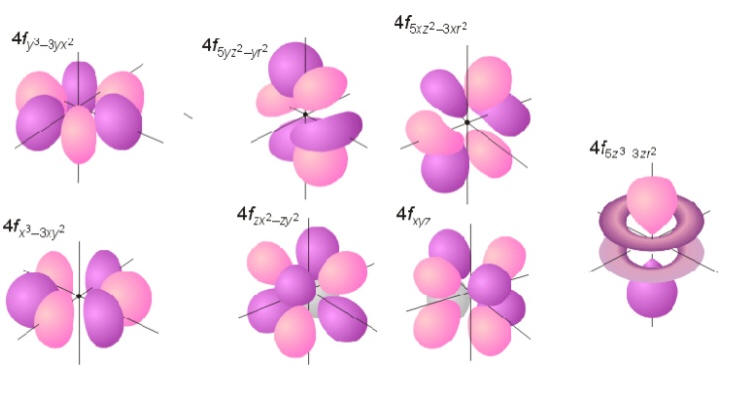
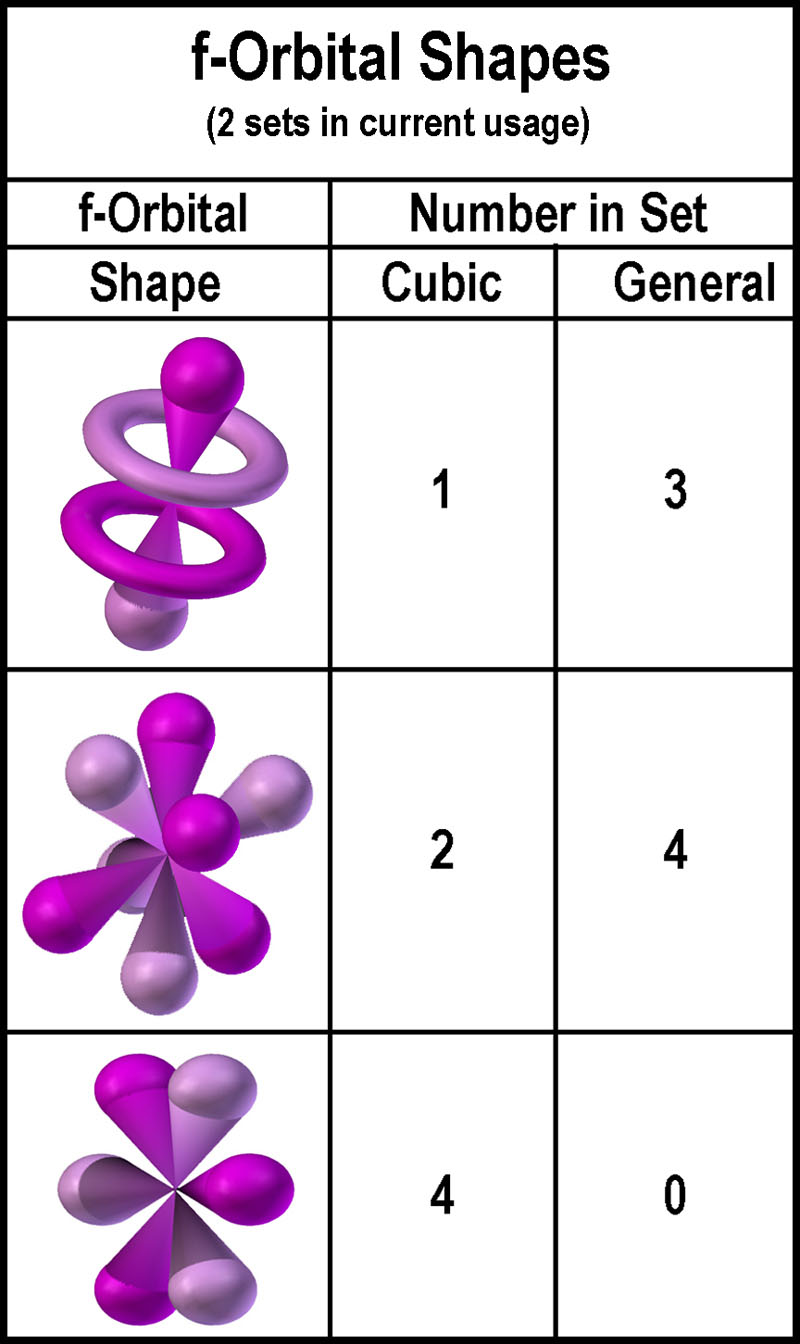
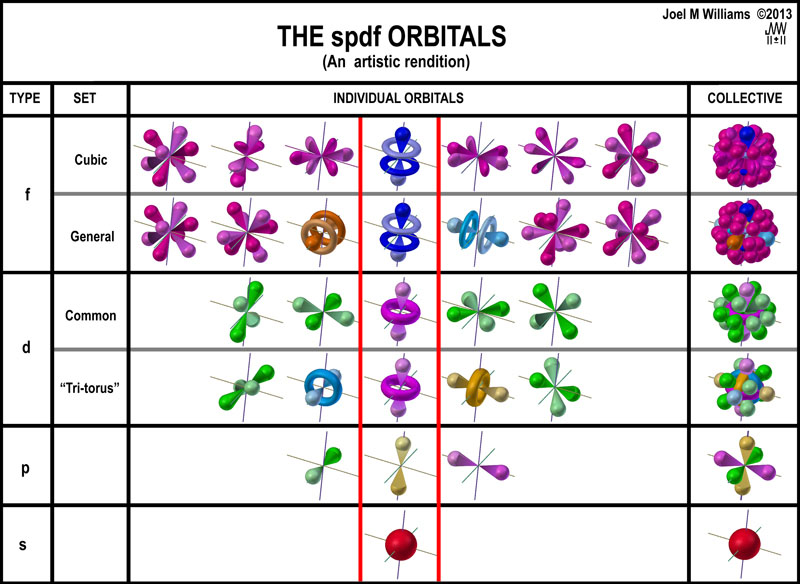
The shapes of p d and f orbitals are described verbally here and shown graphically in the orbitals table below. For d subshell l 2 there are five values of m namely 2 1 0 1 2. You should know that it is impossible to draw an orbital because an electron is capable of taking up all space but we can draw the shape that it takes up most of the time presumably 90 of the time. Means d orbitals can have five orientations.
Shape of d orbitals. They have even more complicated shapes. At the fourth and higher levels there are seven f orbitals in addition to the 4s 4p and 4d orbitals. Of the four we ll be concerned primarily with s and p orbitals because these are the most common in organic chemistry.
Counting the 4s 4p and 4d orbitals this makes a total of 16 orbitals in the fourth level.