S P D F Orbitals How Many Electrons
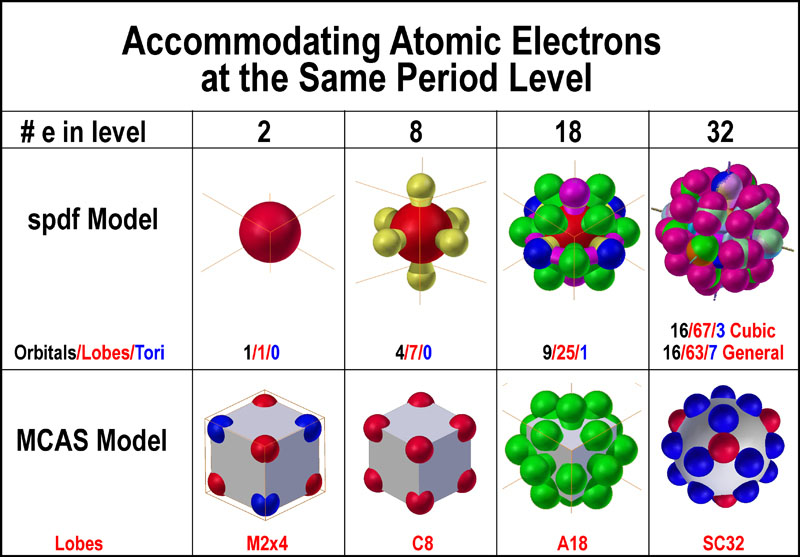
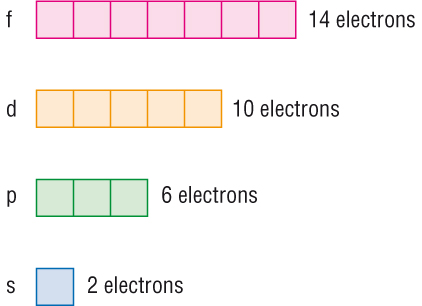
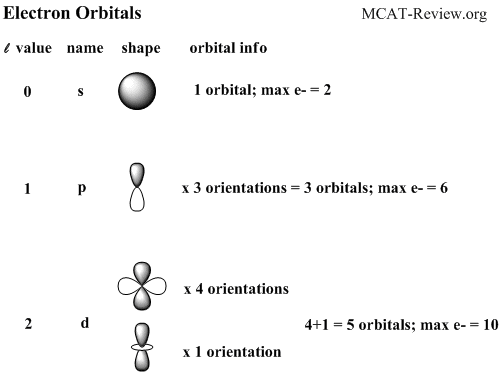
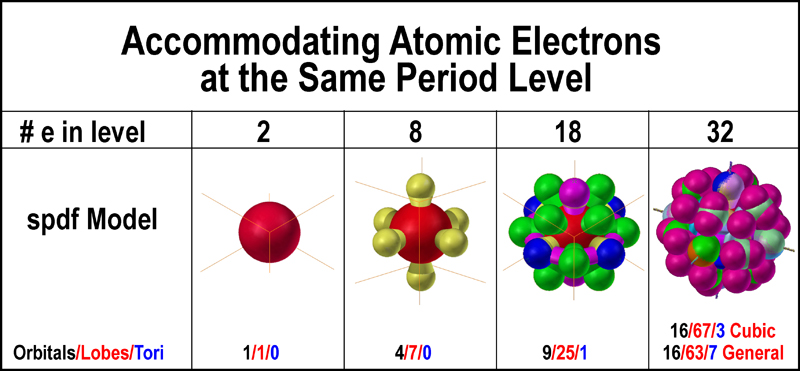
This gives two electrons in an s subshell six electrons in a p subshell ten electrons in a d subshell and fourteen electrons in an f subshell.
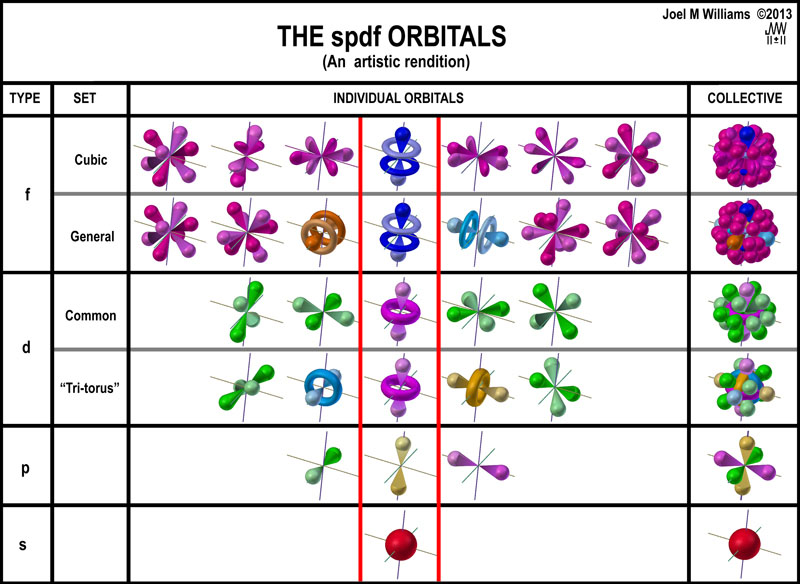
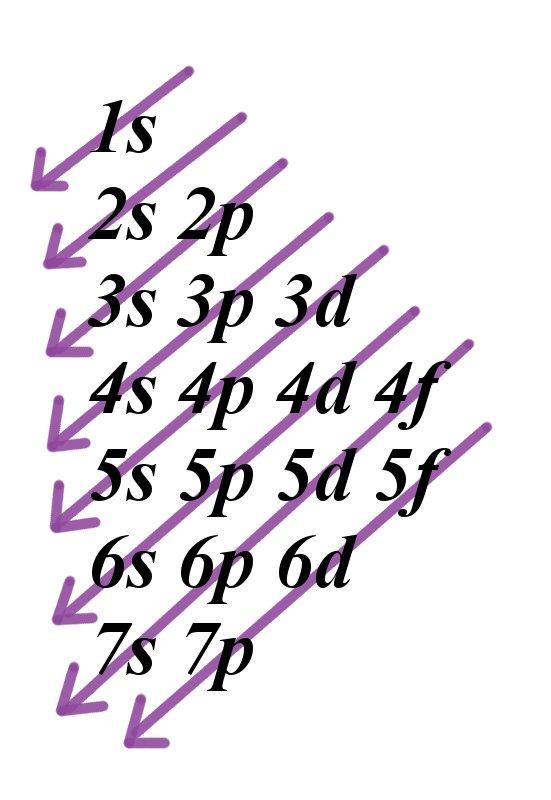
S p d f orbitals how many electrons. The numbers of electrons that can occupy each shell and each subshell arise from the equations of quantum mechanics 2 in particular the pauli exclusion principle which states that no two electrons in the same atom can have the same values of the. D orbitals are transition metals count from sc to zn how many elements are there. At the fourth and higher levels there are seven f orbitals in addition to the 4s 4p and 4d orbitals. S p d and f orbitals are available at all higher energy levels as well.
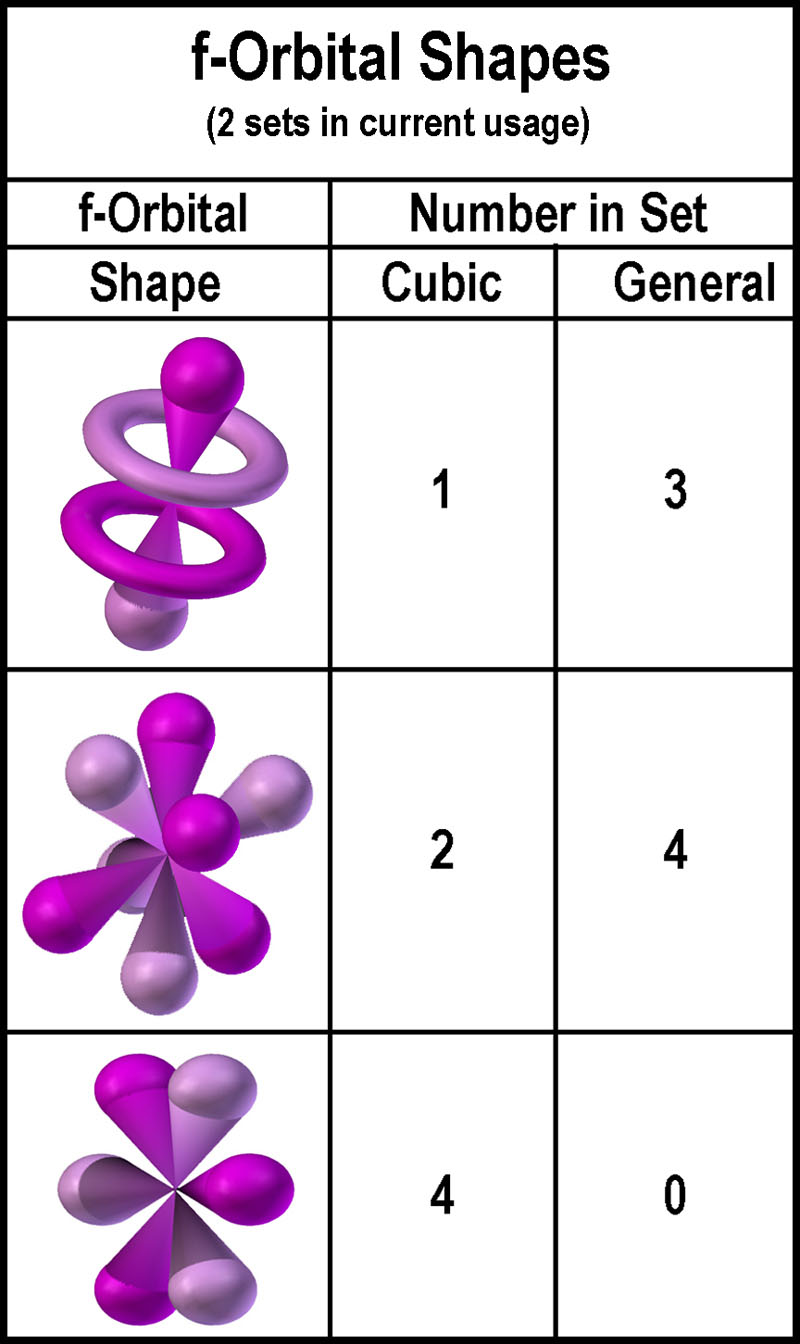
The most complex set of orbitals are the f orbitals. P orbitals are boron noble gas groups. S orbitals are in groups 1 and 2. Counting the 4s 4p and 4d orbitals this makes a total of 16 orbitals in the fourth level.
They have even more complicated shapes. 10 f orbitals is the lanthanides and the actinides count them again and you get 14 elements in a row thats how i remembered the orbitals. Note that all five of the orbitals have specific three dimensional orientations. S 2 electrons p 6 electrons d 10 electrons f 14 electrons each single s orbital has two electrons in it.
7 orbitals 14 electrons. The subshells s p d and f contain the following number of orbitals respectively where every orbital can hold up to two electrons maximum. Each p orbital has two electrons in it and as there are three of these orbitals in. When l 2 m 1 values can be 2 1 0 1 2 for a total of five d orbitals.
1 orbital 2 electrons.